Publications
Peer Reviewed – Submitted
-
- Optogenetic stimulation of midbrain dopamine neurons produces striatal serotonin release M. Dagher, K. A. Perrotta, S. A. Erwin, A. Hachisuka, R. Iyer, S. C. Masmanidis, H. Yang, and A. M. Andrews, ACS Chemical Neuroscience (STATUS: in revision)
2022

-
- Wearable aptamer-field-effect transistor sensing system for noninvasive cortisol monitoring B. Wang, C. Zhao, Z. Wang, K.-A. Yang, X. Cheung, W. Liu, W. Yu, S. Lin, Y. Zhao, K. M. Cheung, H. Lin, H. Hojaiji, P. S. Weiss, M. N. Stojanović, A. J. Tomiyama, A. M. Andrews , and S. Emaminejad, Science Advances 8: eabk0967 (2022). DOI: 10.1126/sciadv.abk0967
2021

-
- Implantable aptamer–field-effect transistor neuroprobes for in vivo neurotransmitter monitoring C. Zhao, K. M. Cheung, I. W. Huang, H. Yang, N. Nakatsuka, W. Liu, Y. Cao, T. Man, P. S. Weiss, H. Monbouquette, and A. M. Andrews , Science Advances Vol. 7, Issue 48 (2021). DOI: DOI: 10.1126/sciadv.abj7422
- Simultaneous serotonin and dopamine monitoring across timescales by rapid pulse voltammetry with partial least squares regression C. S. Movassaghi, K. A. Perrotta, H. Yang, R. Iyer, X. Cheng, M. Dagher, M. Fillol, and A. M. Andrews , Anal Bioanal Chem. 413(27):6747-6767 (2021). DOI: 10.1007/s00216-021-03665-1
- Narrower nanoribbon biosensors fabricated by chemical lift-off lithography show higher sensitivity C. Zhao, Q. Liu, K. M. Cheung, W. Liu, Q. Yang, X. Xu, P. S. Weiss, C. Zhou, and A. M. Andrews, ACS Nano 15:904-915 (2021). DOI: 10.1021/acsnano.0c07503
- Divalent cation dependence enhances dopamine aptamer biosensing. N. Nakatsuka, J. M. Abendroth, K. A, Yang, and A. M, Andrews, ACS Applied Materials & Interfaces (2021). DOI: /10.1021/acsami.0c17535
2020

-
- Flexible Multifunctional In2O3 Nanoribbon Aptamer-Field-Effect Transistor Biosensing Q. Liu, C. Zhao, M. Chen, Y. Liu, Z. Zhao, F. Wu, Z. Li, P. S. Weiss, A. M. Andrews, and C. Zhou, iScience 10.1016/j.isci.2020.101469 (2020).
- Detecting DNA and RNA and Differentiating Single-Nucleotide Variations via Field-Effect Transistors. K. M. Cheung, J. M. Abendroth, N. Nakatsuka, B. Zhu, Y. Yang, A. M. Andrews, and P. S. Weiss, Nano Letters 10.1021/acs.nanolett.0c01971 (2020).
- Scalable fabrication of quasi-one-dimensional Au nanoribbons for plasmonic sensing. C. Zhao, X. Xu, A. R. Ferhan, N. Chiang, J. A. Jackman, Q. Yang, W. Liu, A. M. Andrews, N.-J. Cho, and P. S. Weiss, Nano Letters 20:1747-1754 (2020). PMCID:PMC7067626
- Chemical lift-off lithography of metal and semiconductor surfaces. K. M. Cheung, D. M. Stemer, C. Zhao, T. D. Young, J. N. Belling, A. M. Andrews, and P. S. Weiss, ACS Materials Letters 2:76-83 (2020). PMCID: PMC7220117
2019

-
- Nanoscience and nanotechnology at UCLA. A. Khademhosseini, A. E. Nel, H. Bunje, C. J. DeSantis, A. M. Andrews, R, A. Blaik, Z. Gu, H, Meng, A. Ozcan, S. H. Tolbert, T. Xia, J. I. Zink, and P. S. Weiss. ACS Nano. 13:6127-6129 (2019).
- Phenylalanine Monitoring via Aptamer-Field-Effect Transistor Sensors . K. M. Cheung, K.-A. Yang, N. Nakatsuka, C. Zhao, M. Ye, M. E. Jung, H. Yang, P. S. Weiss, M. N. Stojanović, and A. M. Andrews, ACS Sensors 4:3308-3317 (2019). (Invited cover) PMCID:PMC6957227
- n Utero Exposure to Citalopram Mitigates Maternal Stress Effects on Fetal Brain Development. J. C. Velasquez, Q. Zhao, Y. Chan, L. C. M. Galindo, C. Simasotchi, D. Wu, Z. Hou, S. M. Herod, T. F. Oberlander, S. Gil, T. Fournier, I. Burd, A. M. Andrews, and A. Bonnin, ACS Chemical Neuroscience 10:3307-3317 (2019). PMCID: PMC6733519
- Kappa opioid receptors drive a tonic aversive component of chronic pain. S. S. Liu, S. Pickens, N. E. Burma, I. Ibarra-Lecue, H. Yang, L. Xue, C. Cook, J. K. Hakimian, A. L. Severino, L. Lueptow, K. Komarek, A. M. Taylor, M. C. Olmstead, F. I. Carroll, C. E. Bass, A. M. Andrews, W. Walwyn, R. Trang, C. J. Evans, F. Leslie, C. M. Cahill, Journal of Neuroscience 39:4162-4178 (2019). PMCID: PMC6529867
- Editors’ Favorites: Best of 2018. C. W. Lindsley, C. Abbott, A. M. Andrews, J. M. Hooker, J. Zhou. ACS Chemical Neuroscience 16:1-4 (2019).
2018
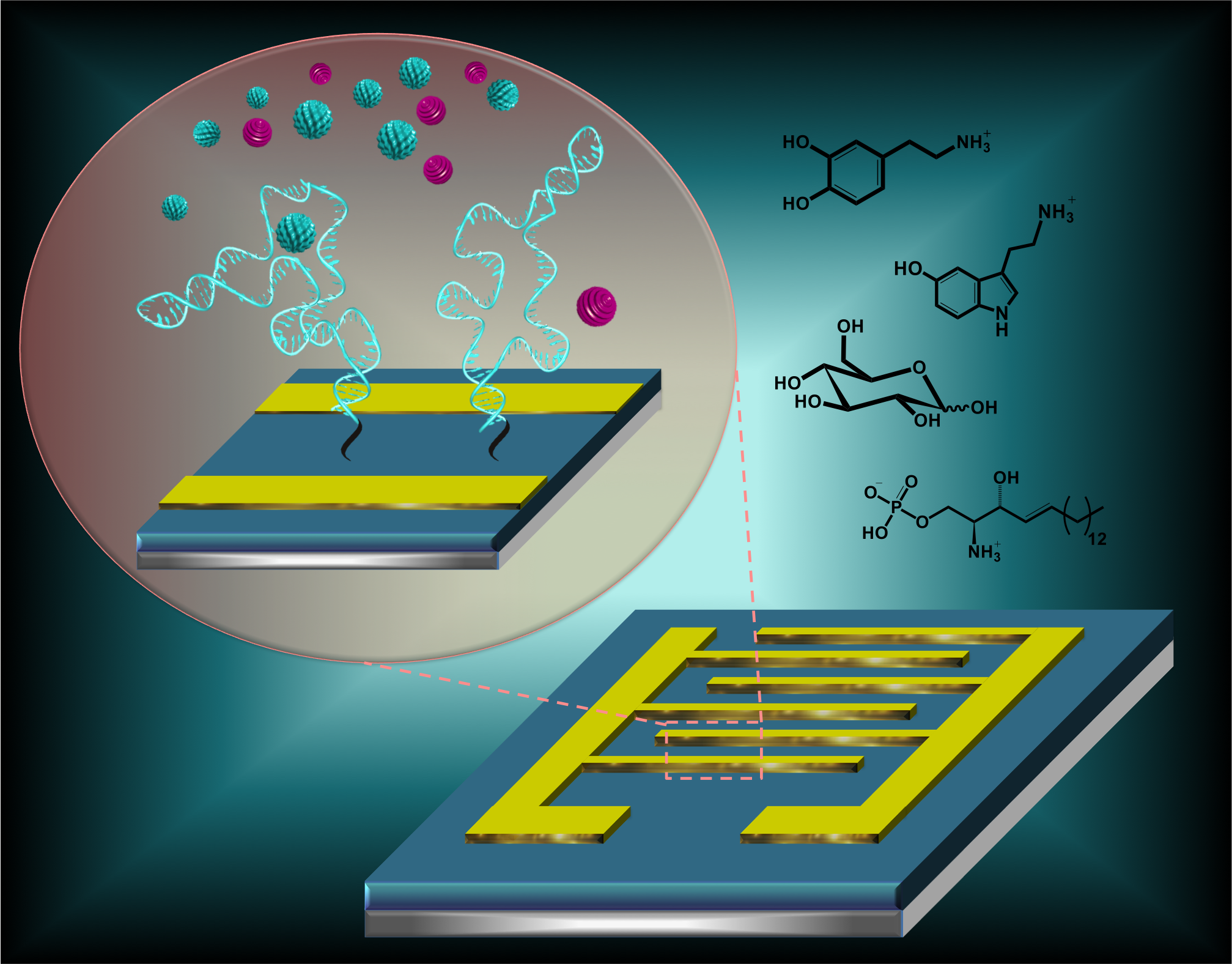


-
- Dark classics in chemical neuroscience: 3,4-Methylenedioxymethamphetamine. L. E. Dunlap, A. M. Andrews, D. E. Olson. ACS Chemical Neuroscience 9:2408-2427 (2018). PMCID: PMC6197894
- Aptamer-field-effect transistors overcome Debye length limitations for small-molecule sensing. N. Nakatsuka, K.-A. Yang, J. M. Abendroth, K. M. Cheung, X. Xu, H. Yang, C. Zhao, B. Zhu, Y. S. Rim, Y. Yang, P. S. Weiss, M. N. Stojanović, and A. M. Andrews, Science 362:319–324 (2018). PMCID: PMC6663484
- Large-area, ultrathin metal-oxide semiconductor nanoribbon arrays fabricated by chemical lift-off lithography. C. Zhao, X. Xu, S.-H. Bae, Q. Yang, W. Liu, J. N. Belling, K. M. Cheung, Y. S. Rim, Y. Yang, A. M. Andrews, and P. S. Weiss, Nano Letters 18:5590–5595 (2018). PMCID: PMC6363913
- Aptamer recognition of multiplexed small-molecule-functionalized substrates. N. Nakatsuka, H. H. Cao, S. Deshayes, A. L. Melkonian, A. M. Kasko, P. S. Weiss, and A. M. Andrews, (2018) ACS Applied Materials & Interfaces 10:23490-23500. PMCID: PMC6087467
- Small-molecule patterning via pre-functionalized alkanethiols. H. H. Cao*, N. Nakatsuka*, S. Deshayes, H. Yang, P. S. Weiss, A. M. Kasko, and A. M. Andrews (*equal contributors), Chemistry of Materials 30:4017-4030 (2018). PMCID: PMC6393937
- Polyserotonin nanoparticles as multifunctional materials for biomedical applications. N. Nakatsuka, M. M. Hasani-Sadrabadi, K. M. Cheung, T. D. Young, G. Bahlakeh, A. Moshaverinia, P. S. Weiss, and A. M. Andrews, ACS Nano 12:4761-4774 (2018).
- In memorium: Dennis L. Murphy, MD.. J. Potash, A. Holmes, and A. M. Andrews, Neuropsychopharmacology 43:1193-1194 (2018).
- Bad behavior: Improving reproducibility in behavior testing. A. M. Andrews, X. Cheng, S. C. Altieri, H. Yang, ACS Chemical Neuroscience 9:1904-1906 (2018). PMCID: PMC6100770
- Editors’ Favorites of 2017. C. W. Lindsley, K. A. Cunningham, J. M. Hooker, A. M. Andrews, ACS Chemical Neuroscience 9:1-4 (2018).
2017


-
- Patterning of supported gold monolayers via chemical lift-off lithography. L. S. Slaughter, K. M. Cheung, S. Kaappa, H. H. Cao, Q. Yang, T. D. Young, A. C. Serino, S. Malola, J. M. Olson, S. Link, H. Häkkinen, A. M. Andrews, and P. S. Weiss, Beilstein Journal of Nanotechnology 8:2648-2661 (2017).
- High-affinity nucleic-acid based receptors for steroids. K.-A. Yang, H.-S. Chun, Y. Zhang, S. Pecic, N. Nakatsuka, A. M. Andrews, T. P. Worgall, and M. N. Stojanović, ACS Chemical Biology 12:3103-3112 (2017).
- Advancing biocapture substrates via chemical lift-off lithography. H. H. Cao, W.-S. Liao, A. Serino, H. Yang, P. S. Weiss, and A. M. Andrews, Chemistry of Materials 29:6829–6839 (2017).
- Self-collapse lithography. C. Zhao, X. Xu, Q. Yang, T. Man, S. J. Jonas, J. J. Schwartz, A. M. Andrews, and P. S. Weiss, Nano Letters 17:5035-5042 (2017).
- Analyzing spin selectivity in DNA-mediated charge transfer via fluorescence microscopy. J. M. Abendroth, N. Nakatsuka, M. Ye, D. Kim, E. E. Fullerton, A. M. Andrews, and P. S. Weiss, ACS Nano 11:7516-7526 (2017).
- Emerging trends in micro- and nanoscale technologies in medicine: From basic discoveries to translation. M. M. Alvarez, J. Aizenberg, M. Analoui, A. M. Andrews, G. Bisker, E. S. Boyden, R. D. Kamm, J. M. Karp, D. J. Mooney, R. Oklu, D. Peer, M. Stolzoff, M. S. Strano, G. Trujillo-de Santiago, T. J. Webster, P. S. Weiss, and A. Khademhosseini, ACS Nano 11:5195-5214 (2017).
- The chemistry of thought: The role of the measurement sciences in brain research. Andrews, A. M., Bhargava, R., Kennedy, R., Li, L., and Sweedler, J. V., Analytical Chemistry 89:4757 (2017).
- Polymer-pen chemical lift-off lithography. X. Xu, Q. Yang, K. M. Cheung, C. Zhao, N. Wattanatorn, J. N. Belling, J. M. Abendroth, L. S. Slaughter, C. A. Mirkin, A. M. Andrews, and P. S. Weiss, Nano Letters 17:3302-3311 (2017).
- Interplay between materials and microfluidics. X. Hou, Y. S. Zhang, G. Trujillo-de Santiago, M. M. Alvarez, J. Ribas, S. J. Jonas, P. S. Weiss, A. M. Andrews, J. Aizenberg, and A. Khademhoussini, Nature Reviews|Materials 2:17016 (2017), cover.
- Diverse applications of nanomedicine. B. Pelaz, C. Alexiou, R. A. Alvarez Puebla, F. Alves, A. M. Andrews, S. Ashraf, L. P. Balogh, L. Ballerini, A. Bestetti, C. Brendel, S. Bosi, M. Carril, W. C. W. Chan, C. Chen, X. Chen, X. Chen, Z. Cheng, D. Cui, J. Du, C. Dullin, A. Escudero, N. Feliu, M. Gao, M. George, A. Grünweller, Z. Gu, Y. Gogotsi, N. J. Halas, N. Hampp, R. K. Hartmann, M. C. Hersam, P. Hunziker, J. Jian, X. Jiang, P. Jungebluth, P. Kadhiresan, K. Kataoka, A. Khademhosseini, J. Kopecek, N. A. Kotov, H. F. Krug, D. S. Lee, C.-M. Lehr, K. W. Leong, X.-J. Liang, M. Lim, Luis M. Liz Marzán, X. Ma, P. Macchiarini, H. Meng, H. Möhwald, P. Mulvaney, A. E. Nel, S. Nie, P. Nordlander, T. Okano, J. Oliveira, T. H. Park, R. M. Penner, M. Prato, V. Puntes, V. Rotello, A. Samarakoon, R. E. Schaak, Y. Shen, S. Sjoqvist, A. G. Skirtach, M. G. Soliman, M. M. Stevens, B. Z. Tang, R. Tietze, S. Van Epps, B. N. Udugama, H.-W. Sung, T. Weil, P. S. Weiss, I. Willner, Y. Wu, L. Yang, Z. Yue, Q. Zhang, Q. Zhang, X.-E. Zhang, Y. Zhao, X. Zhou, and W. J. Parak, ACS Nano 11:2313-2381 (2017) Editors’ Choice.
- Differentiating siblings: The case of dopamine and norepinephrine. N. Nakatsuka and A. M. Andrews, ACS Chemical Neuroscience 8:218-220 (2017).
- Why monitor molecules in neuroscience?. M. Andrews, ACS Chemical Neuroscience 8:211-212 (2017).
- Editors’ favorites of 2016. C. W. Lindsley, J. M. Hooker, K. A. Cunningham, A. M. Andrews, ACS Chemical Neuroscience 8:1-3 (2017).
- Advanced microdialysis approaches resolve differences in serotonin homeostasis and signaling. M. M. Sampson, H. Yang, and A. M. Andrews in Compendium of In-Vivo Monitoring in Real-Time Molecular Neuroscience. A. C. Michael, ed. Vol. 2, World Scientific Publishing, Hackensack, NJ (2017). http://www.worldscientific.com/worldscibooks/10.1142/10462
2016

-
- A model for teaching advanced neuroscience methods: A student-run seminar to increase practical understanding and confidence. T. M. Harrison, C. R. K. Ching, and A. M. Andrews, Journal of Undergraduate Neuroscience Education 15:A5-A10 (2016). PMCID: PMC5105964
- Double-sided opportunities using chemical lift-off lithography. A. M. Andrews, W.-S. Liao, and P. S. Weiss, Accounts of Chemical Research 49:1449-1457 (2016).

2015


-
- Neurochips enable nanoscale devices for high resolution in vivo neurotransmitter sensing. N. Nakatsuka and A. M. Andrews, Neuropsychopharmacology 41:378-379 (2016).
- Printable ultrathin metal oxide semiconductor-based conformal biosensors. Printable ultrathin metal oxide semiconductor-based conformal biosensors. Y. S. Rim, S.-H. Bae, H. Chen, J. L. Yang, J. Kim, A. M. Andrews, P. S. Weiss, Y. Yang, and H.-R. Tseng, ACS Nano 9:12174-12181 (2015).
- Controlled DNA patterning by chemical lift-off lithography: Matrix matters. H. H. Cao, W.-S. Liao, A. Serino, N. Nakatsuka, S. Cheunkar, H. Yang, P. S. Weiss, and A. M. Andrews, (2015) ACS Nano 9:11439-11454 (2015).
- Serotonin states and social anxiety. M. B. Stein and A. M. Andrews, JAMA Psychiatry, 72:845-847 (2015).
- Prefrontal cortex vistas: A serotonin safari. A. M. Andrews, ACS Chemical Neuroscience 6:936-937 (2015).
- Sex- and SERT-associated differences in stimulated serotonin revealed by fast microdialysis. H. Yang, M. M. Sampson, D. Senturk, and A. M. Andrews, ACS Chemical Neuroscience 6:1487-1501 (2015).
- Perinatal vs. genetic programming of serotonin states associated with anxiety genetic programming of serotonin states associated with anxiety. genetic programming of serotonin states associated with anxiety. S. C. Altieri, H. Yang, H. J. O’Brien, H. M. Redwine, D. Senturk, J. G. Hensler, and A. M. Andrews, Neuropsychopharmacology 40:1456-1470 (2015). PMCID: PMC4397404
- Fabrication of high-performance ultrathin In2O3 film field-effect transistors and biosensors using chemical lift off lithography. J. Kim, Y. S. Rim, H. Chen, H. H. Cao, N. Nakatsuka, H. L. Hinton, C. Zhao, A. M. Andrews, Y. Yang, and P. S. Weiss, ACS Nano 9:4572–4582 (2015).
- The future of monitoring molecules. A. M. Andrews, ACS Chemical Neuroscience, 6:1-2 (2015).
- Flow cytometry to determine serotonin transporter function in human peripheral blood cells. B. S. Beikmann and A. M. Andrews in Serotonin Receptor Technologies. W. Blenau and A. Baumann, eds., Neuromethods series, W. Walz, Editor-in-chief, Springer, New York, NY (2015). ISBN: 978-1-4939-2186-7 (Print) 978-1-4939-2187-4 (Online). http://link.springer.com/book/10.1007/978-1-4939-2187-4
- Electrochemical techniques and advances in psychopharmacology. L. C. Daws, A. M. Andrews, and G. A. Gerhardt, in Encyclopedia of Psychopharmacology, I. P. Stolerman, ed., Springer, New York, NY pp. 586 592 (2015). SBN: 978-3-642-36171-5 (Print) 978-3-642-36172-2 (Online). http://link.springer.com/referenceworkentry/10.1007/978-3-642-36172-2_311
2014
-
- Functional characterization of the S41Y (C2755A) polymorphism of tryptophan hydroxylase 2. N. Carkaci-Salli, U. Salli, I. Tekin, M. K. Zhao, T. L. Gilman, A. M. Andrews, and K. E. Vrana, Journal of Neurochemistry 130:748-758 (2014).
- 2’-NH2-MPTP: A serotonin and norepinephrine neurotoxin. J. B. Ochroch, A. J. Bressler, H. Yang, D. L. Murphy, S. C. Altieri, and A. M. Andrews in Handbook of Neurotoxicity. R. Kostrzewa, ed., Springer, New York, NY (2014). ISBN: 978-1-4614-5835-7 (Print) 978-1-4614-5836-4 (Online). http://link.springer.com/referencework/10.1007/978-1-4614-5836-4
- Chemistry and the BRAIN Initiative. A. M. Andrews, A. Schepartz, J. V. Sweedler, and P. S. Weiss, Journal of the American Chemical Society 136:1-2 (2014).
2013

-
- The BRAIN initiative: Toward a chemical connectome. A. M. Andrews, ACS Chemical Neuroscience 4:645 (2013).
- What’s old is new. A. M. Andrews and L. C. Daws, ACS Chemical Neuroscience 4:1-2 (2013).
- Nanotools for neuroscience and brain activity mapping. A. P. Alivisatos, A. M. Andrews, E. S. Boyden, M. Chun, G. M. Church, K. Deisseroth, J. P. Donoghue, S. E. Fraser, J. Lippincott-Schwartz, L. L. Looger, S. Masmanidis, P. L. McEuen, A. V. Nurmikko, H. Park, D. S. Peterka, C. Reid, M. L. Roukes, A. Scherer, M. Schnitzer, T. J. Sejnowski, K. L. Shepard, D. Tsao, G. Turrigiano, P. S. Weiss, C. Xu, R. Yuste, X. Zhuang, ACS Nano 7:1850-1866 (2013) PMCID: PMC3665747.
- Rethinking serotonin 1A receptors: Emerging modes of inhibitory feedback of relevance to emotion-related behavior. S. C. Altieri, A. L. Garcia-Garcia, E. D. Leonardo, and A. M. Andrews, ACS Chemical Neuroscience 4:72-83 (2013) PMCID: PMC354747.
- From the bottom up: Dimensional control and characterization in molecular monolayers. S. A. Claridge, W.-S. Liao, J. C. Thomas, Y. Zhao, H. H. Cao, S. Cheunkar, A. C. Serino, A. M. Andrews, and P. S. Weiss, Chemical Society Reviews 42:2725-2745 (2013).
- Functional common promoter and rare coding region variants in the serotonin transporter gene, SLC6A4, associated with Tourette disorder. P. R. Moya, J. R. Wendland, A. M. Andrews, L. M. Rubenstein, K. R. Timpano, G. A. Heiman, J. A. Tischfield, R. A. King, S. Ramamoorthy, F. J. McMahon, and D. L. Murphy, Movement Disorders 28:1263-70 (2013). PMCID: PMC3766488
- Small-molecule arrays for sorting G-protein-coupled receptors. W.-S. Liao, H. H. Cao, S. Cheunkar, M. J. Shuster, S. C. Altieri, P. S. Weiss, and A. M. Andrews, Journal of Physical Chemistry C 117:22362–22368 (2013).
- Physiologically relevant changes in serotonin resolved by fast microdialysis. H. Yang, A. B. Thompson, B. J. McIntosh, S. C. Altieri, and A. M. Andrews, ACS Chemical Neuroscience 4:790-798 (2013). PMCID: PMC3656759
- The real catecholamine content of secretory vesicles in the CNS revealed by electrochemical cytometry. D. M. Omiatek, A. J. Bressler, A.–S. Cans, A. M. Andrews, M. L. Heien, and A. G. Ewing, Scientific Reports 3:1447 (2013) PMCID: PMC3596796.
- Serotonin uptake is largely mediated by platelets versus lymphocytes in peripheral blood cells. B. S. Beikmann, I. D. Tomlinson, S. J. Rosenthal, and A. M. Andrews, ACS Chemical Neuroscience 4:161-170 (2013). PMCID: PMC3547482
2012

-
- Subtractive patterning via chemical lift-off lithography. W.–S. Liao, S. Cheunkar, H. H. Cao, H. R. Bednar, P. S. Weiss, and A. M. Andrews, Science 327:1517-1521 (2012).
- Nano in the brain: Nano-neuroscience. A. M. Andrews and P. S. Weiss, ACS Nano 6:8463-8464 (2012).
- Celebrating serotonin. A. M. Andrews, ACS Chemical Neuroscience 3:644–645 (2012).
- Visual strategies and cover art. A. M. Andrews, ACS Chemical Neuroscience 3:492 (2012).
- Serotonergic pathways in depression. S. C. Altieri, Y. S. Singh, E. Sibille, and A. M. Andrews in Neurobiology of Depression. F. López-Muñoz and C. Álamo, eds. Frontiers in Neuroscience Book Series, Boca Raton, FL (2012).
- Differential serotonin transport is linked to the rh5 HTTLPR in peripheral blood cells. *Y. S. Singh, *S. C. Altieri, T. L. Gilman, H. A. Michael, I. D. Tomlinson, S. J. Rosenthal, G. M. Swain, M. A. Murphy-Corb, R. E. Ferrell, and A. M. Andrews, Translational Psychiatry 2: e77 (2012) PMCID: PMC3309549 *joint first-authors.
2011


-
- Comparison of oligo(ethylene glycol)alkanethiols vs n-alkanethiols: Self-assembly, insertion, and functionalization. M. J. Shuster, A. Vaish, M. L. Gilbert, M. Martinez-Rivera, R. M. Nezarati, P. S. Weiss, and A. M. Andrews, Journal of Physical Chemistry C 115: 24778–24787 (2011).
- Thin gold film-assisted fluorescence spectroscopy for biomolecule sensing. A. Vaish, W.-S. Liao, M. J. Shuster, J. M. Hinds, P. S. Weiss, and A. M. Andrews, Analytical Chemistry 83: 7451–7456 (2011).
- Patterning small-molecule biocapture surfaces: Microcontact insertion printing vs. photolithography. photolithography. M. J. Shuster, A. Vaish, H. H. Cao, A. I. Guttentag, J. E. McManigle, A. L. Gibb, M. Martinez-Rivera, R. M. Nezarati, J. M. Hinds, W.-S. Liao, P. S. Weiss, and A. M. Andrews, Chemical Communications 47:10641–10643 (2011).
- Head-to-head comparisons of carbon fiber microelectrode coatings for sensitive and selective neurotransmitter detection by voltammetry. Y. S. Singh, L. A. Sawarynski, P. D. Dabiri, W. R. Choi, and A. M. Andrews, Analytical Chemistry 83:6658-6666 (2011). PMCID: PMC3165139
- Tuning stamp surface energy for soft lithography of polar molecules to fabricate small-molecule microarrays. A. Vaish, M. J. Shuster, S. Cheunkar, P. S. Weiss, and A. M. Andrews, Small 7:1471-1479 (2011), cover.
2010


-
- Electrochemical techniques and advances in psychopharmacology. L. C. Daws, A. M. Andrews, and G. A. Gerhardt, in Encyclopedia of Psychopharmacology, I. P. Stolerman, ed., Springer, New York, NY pp. 458 462(2010). http://link.springer.com/referenceworkentry/10.1007/978-3-540-68706-1_311
- Presynaptic adaptive responses to constitutive versus adult pharmacologic inhibition of serotonin uptake. B. A. Luellen, T. L. Gilman, and A. M. Andrews, in Experimental Models in Serotonin Transporter Research, A. Kaleuv, ed., Oxford University Press, New York, NY (2010).
- Capillary UHPLC with elevated temperature for sub-one minute separations of basal serotonin in sub-microliter brain microdialysate samples. Y. Liu, J. Zhang, X. Xu, M. K. Zhao, A. M. Andrews, and S. G. Weber, Analytical Chemistry 82:9611-9616 (2010) PMCID: PMC3008768.
- Biomarkers to predict antidepressant response. A. F. Leuchter, I. A. Cook, S. P. Hamilton, K. L. Narr, A. Toga, A. M. Hunter, K. Faull, J. Whitelegge, A. M. Andrews, J. Loo. B. Way. S. F. Nelson, S. Horvath, and B. D. Lebowitz, Current Psychiatry Reports 12:553-562 (2010). PMCID: PMC2965366
- γ‐Aminobutyric acid‐type A receptor deficits cause hypothalamic‐pituitary‐adrenal axis hyperactivity and antidepressant drug sensitivity reminiscent of melancholic forms of depression. Q. Shen, R. Lal, B. A. Luellen, J. C. Earnheart, A. M. Andrews, and B. Lüscher, Biological Psychiatry 68:512‐520 (2010) PMCID: PMC2930197.
- Native serotonin membrane receptors recognize 5-hydroxytryptophan-functionalized substrates: Enabling small-molecule recognition. A. Vaish, M. J. Shuster, S. Cheunkar, Y. S. Singh, P. S. Weiss, and A. M. Andrews, ACS Chemical Neuroscience 1:495-504 (2010) PMCID: PMC3368647.
- Low-stress route learning using the Lashley III maze in young and aging mice. A. Bressler, D. Blizard, and A. Andrews, Journal of Visualized Experiments 39: http://www.jove.com/index/details.stp?id=1786 (2010). PMCID: PMC3284847
- Boron-doped diamond microelectrodes reveal reduced rates of serotonin uptake in lymphocytes from adult rhesus monkeys carrying the short allele of the 5-HTTLPR. Y. S. Singh, L. E. Sawarynski, H. A. Michael, R. E. Ferrell, M. A. Murphy-Corb, G. M. Swain, B. A. Patel, and A. M. Andrews, ACS Chemical Neuroscience 1:49-64 (2010) PMCID: PMC2843923.
2009
-
- Does chronic antidepressant treatment increase extracellular serotonin?. A. M. Andrews, perspective for Frontiers in Neuroscience (Depression special issue) 3:246-247 (2009).
2008
-
- How the serotonin story is being rewritten by new gene-based discoveries principally related to SLC6A4, the serotonin transporter gene, which functions to influence all cellular serotonin systems. D. L. Murphy, M. A. Fox, K. R. Timpano, P. Moya, R. Ren-Patterson, A. M. Andrews, A. Holmes, S. W. Watts. J. R. Wendland, and K.-P. Lesch, Neuropharmacology 55:932-960 (2008) PMCID: PMC2730952
- Hybrid approaches to nanometer-scale patterning: Exploiting tailored intermolecular interactions. T. J. Mullen, C. Srinivasan, M. J. Shuster, M. W. Horn, A. M. Andrews, and P. S. Weiss, Journal of Nanoparticle Research 10:1231-1240 (2008).
- Biospecific recognition of tethered small molecules diluted in self-assembled monolayers. M. J. Shuster, A. Vaish, M. E. Szapacs, M. E. Anderson, P. S. Weiss, and A. M. Andrews, Advanced Materials 20:164-167 (2008).
- Brain-derived neurotrophic factor-deficient mice exhibit a hippocampal hyperserotonergic phenotype. B. P. Guiard, D. P. David, T. Deltheil, J.–P. Guilloux, F. Chenu, E. Le Maître, T. Renoir, I. Leroux-Nicollet, P. Sokoloff, V. Arango, Y. Liu, M. Underwook, L. Lanfumey, M. Hamon, A. M. Andrews, R. Hen, and A. M. Gardier, International Journal of Neurospychopharmacology 11:79-92 (2008).
2007

-
- Selecting and driving monolayer structures through tailored intermolecular interactions. T. J. Mullen, A. A. Dameron, A. M. Andrews, and P. S. Weiss, Aldrichimica Acta 40:21-31 (2007) invited, cover.
- Determining serotonin and dopamine uptake rates using high-speed chronoamperometry. X. A. Perez, A. J. Bressler, and A. M. Andrews, in Electrochemical Methods for Neuroscience. A. C. Michael and L. A. Borland, eds., CRC Press LLC, Boca Raton, FL, 103-124 (2007).
- A pharmacological analysis of mice with a targeted disruption of the serotonin transporter. M. A. Fox, A. M. Andrews, J. R. Wendland, K.–P. Lesch, A. Holmes, and D. L. Murphy, Psychopharmacology 195:147-66 (2007).
- Scanning electron microscopy of nanoscale chemical patterns. C. Srinivasan, T. J. Mullen, J. N. Hohman, M. E. Anderson, A. A. Dameron, A. M. Andrews, E. C. Dickey, M. W. Horn, P. S. Weiss, ACS Nano 1:191-201 (2007).
- Reduced BDNF is associated with a loss of serotonergic innervation in the hippocampus of aging mice. B. A. Luellen, L. E. Bianco, L. M. Schneider, and A. M. Andrews, Genes, Brain and Behavior 6:482-490 (2007).
- Microcontact insertion printing. T. J. Mullen, C. Srinivasan, J. N. Hohman, S. D. Gillmor, M. J. Shuster, M. W. Horn, A. M. Andrews, and P. S. Weiss, Applied Physics Letters 90:063114 (1-3) (2007), cover.
2006

-
- Radical-induced degradation of liposome-encapsulated microtubules as a model of axonal damage due to oxidative stress. A. E. Counterman, T. G. D’Onofrio, A. M. Andrews, and P. S. Weiss, Proceedings of the National Academy of Sciences 103:5262-5266 (2006), cover PMCID: PMC1459344.
- Filtration disrupts synaptosomal membranes during radiochemical assay of serotonin uptake: Comparison with chronoamperometry in SERT knockout mice. X. A. Perez, L. E. Bianco, and A. M. Andrews, Journal of Neuroscience Methods 154:245-255 (2006).
- The neurotoxin 2′-NH2-MPTP degenerates serotonin axons and evokes increases in hippocampal BDNF. B. A. Luellen, M. E. Szapacs, C. K. Materese, and A. M. Andrews, Neuropharmacology 50:297-308 (2006).
- Locomotor hyperactivity and alterations in dopamine neurotransmission are associated with overexpression of A53T mutant human a-synuclein in mice. E. L. Unger, D. Eve, X. A. Perez, D. K. Reichenbach, Y. Xu, M. K. Lee, and A. M. Andrews, Neurobiology of Disease 21:431-443 (2006).
2005
-
- Altered serotonin synthesis, turnover, and dynamic regulation in multiple brain regions of mice lacking the serotonin transporter. D.-K. Kim, T. J. Tolliver, S. J. Huang, B. J. Martin, A. M. Andrews, C. Wichems, A. Holmes, K.-P. Lesch, and D. L. Murphy, Neuropharmacology 49:798-810 (2005).
- Chronoamperometry detects differential changes in synaptosomal uptake in serotonin transporter knockout mice. X. A. Perez and A. M. Andrews, Analytical Chemistry 77:818-826 (2005).
2004
-
- Gene dose-dependent alterations in extraneuronal serotonin but not dopamine in mice with reduced serotonin transporter expression. T. A. Mathews, D. E. Fedele, F. M. Coppelli, A. L. Avila, D. L. Murphy, and A. M. Andrews, Journal of Neuroscience Methods 140:169-181 (2004).
- Exploring the relationship between serotonin and brain-derived neurotrophic factor: Analysis of BDNF protein and extraneuronal 5-HT in mice with reduced serotonin transporter or BDNF expression. M. E. Szapacs, T. A. Mathews, L. Tessarollo, W. E. Lyons, L. A. Mamounas, and A. M. Andrews, Journal of Neuroscience Methods 140:81-92 (2004).
- Late onset loss of hippocampal 5-HT and NE is accompanied by an increase in BDNF protein expression in mice co-expressing mutant APP and PS1. M. E. Szapacs, A. L. Numis, and A. M. Andrews, Neurobiology of Disease 16:572-580 (2004).
- The role of membrane and vesicular monoamine transporters in the neurotoxic and hypothermic effects of 2′-NH2-MPTP. A. L. Numis, E. L. Unger, D. L. Sheridan, A. C. Chisnell, and A. M. Andrews, Molecular Pharmacology 66:718-727 (2004).
2003

-
- Neuronal and astroglial responses to the serotonin and norepinephrine neurotoxin: 1-Methyl-4-(2′-aminophenyl)-1,2,3,6-tetrahydropyridine. B. A. Luellen, D. B. Miller, A. C. Chisnell, D. L. Murphy, J. P. O’Callaghan, and A. M. Andrews, Journal of Pharmacology and Experimental Therapeutics 307:923-931 (2003).
2002

-
- 2′-NH2-MPTP (1-Methyl-4-(2′-aminophenyl)-1,2,3,6 tetrahydropyridine) depletes serotonin and norepinephrine in rats: A comparison with 2′-CH3-MPTP (1-methyl-4-(2′-methylphenyl)-1,2,3,6-tetrahydropyridine). E. L. Unger, P. Mazzola-Pomietto, D. L. Murphy, and A. M. Andrews, Journal of Pharmacology and Experimental Therapeutics 303:527-533 (2002).
- GAP-43 is critical for normal development of the serotonergic innervation in forebrain. S. L. Donovan, L. A. Mamounas, A. M. Andrews, M. E. Blue, and J. S. McCasland, Journal of Neuroscience 22:3543-3552 (2002).
2001
-
- Genetic perspectives on the serotonin transporter. D. L. Murphy, Q. Li, S. Engel, C. Wichems, A. M. Andrews, K.-P. Lesch, and G. Uhl, Brain Research Bulletin 56:487-494 (2001).
- Molecular mechanisms of cocaine reward: Combined dopamine and serotonin transporter knockouts eliminate cocaine place preference. I. Sora, F. S. Hall, A. M. Andrews, M. Itokawa, X. F. Li, H. B. Wei, C. Wichems, K.-P. Lesch, D. L. Murphy, and G. R. Uhl, Proceedings of the National Academy of Sciences 98:5300-5305 (2001). PMCID: PMC33204
2000
-
- The use of 2′-NH2-MPTP as a tool to probe central serotonin and norepinephrine neurotransmitter systems. A. M. Andrews, Neurotransmissions 16:18-21 (2000) invited. (PDF can be requested directly from anne.andrews[at]ucla.edu).
1999
-
- Overexpression of human copper/zinc superoxide dismutase in transgenic mice attenuates oxidative stress caused by methylenedioxymethamphetamine (Ecstasy). S. Jayanthi, B. Ladenheim, A. M. Andrews, and J. L. Cadet, Neuroscience 91:1379-1387 (1999).
1998

-
- Brain serotonin neurotransmission: An overview and update with an emphasis on serotonin subsystem heterogeneity, multiple receptors, interactions with other neurotransmitter systems, and consequent implications for understanding the actions of serotonergic drugs. D. L. Murphy, A. M. Andrews, C. H. Wichems, Q. Li, M. Tohda, and B. Greenberg, Journal of Clinical Psychiatry 59:4-12 (1998).
- Delta opioid peptide [D-Ala2,D-leu5]enkephalin blocks the long-term loss of dopamine transporters induced by multiple administrations of methamphetamine: Involvement of opioid receptors and reactive oxygen species. L.-I. Tsao, B. Ladenheim, A. M. Andrews, C. C. Chiueh, J. L. Cadet, and T.-P. Su, Journal of Pharmacology and Experimental Therapeutics 287:322-331 (1998).
- Altered brain serotonin homeostasis and locomotor insensitivity to 3,4 methylenedioxy-methamphetamine (“Ecstasy”) in serotonin transporter-deficient mice. D. Bengel, D. L. Murphy, A. M. Andrews, C. H. Wichems, D. Feltner, A. Heils, R. Mossner, H. Westphal, and K.-P. Lesch, Molecular Pharmacology 53:649-655 (1998).
1997
-
- Cellular localization and expression of the serotonin transporter in mouse brain. D. Bengel, O. Jöhren, A. M. Andrews, A. Heils, R. Mößner, G. L. Sanvitto, J. M. Saavedra, K.-P. Lesch, and D. L. Murphy, Brain Research 778:338-345 (1997).
- Gene structure and 5′-flanking regulatory region of the murine serotonin transporter. D. Bengel, A. Heils, S. Petri, M. Seemann, K. Glatz, A. M. Andrews, D. L. Murphy, and K.-P. Lesch, Molecular Brain Research 44:286-292 (1997).
1996
-
- Differential reinforcing effects of cocaine and GBR-12909: Biochemical evidence for divergent neuroadaptive changes in the mesolimbic dopaminergic system. S. R. Tella, B. Ladenheim, A. M. Andrews, S. R. Goldberg, and J. L. Cadet, Journal of Neuroscience 16:7416-7427 (1996).
- Transgenic mice with high levels of superoxide dismutase activity are protected from the neurotoxic effects of 2′-NH2-MPTP on serotonergic and noradrenergic nerve terminals. A. M. Andrews, B. Ladenheim, C. J. Epstein, J. L. Cadet, and D. L. Murphy, Molecular Pharmacology 50:1511-1519 (1996).
1993
-
- Fluoxetine and desipramine selectively attenuate 2′-NH2-MPTP-induced depletions in serotonin and norepinephrine. A. M. Andrews and D. L. Murphy, European Journal of Pharmacology 250:215-221 (1993).
- 2′-NH2-MPTP in Swiss Webster mice: Evidence for long term (six month) depletions in cortical and hippocampal 5-HT and NE, differential protection by selective uptake inhibitors or clorgyline, and functional changes in central 5-HT neurotransmission. A. M. Andrews and D. L. Murphy, Journal of Pharmacology and Experimental Therapeutics 267:1432-1439 (1993).
- Sustained depletion of cortical and hippocampal serotonin and norepinephrine but not striatal dopamine by 1-methyl-4-(2′-aminophenyl)-1,2,3,6-tetrahydropyridine (2′-NH2-MPTP): A comparative study with 2′-CH3-MPTP and MPTP. A. M. Andrews and D. L. Murphy, Journal of Neurochemistry 60:1167-1170 (1993).